Understanding Graham’s Law and the Need for Containment
Graham’s Law Overview
Graham’s Law describes the relationship between the rate of effusion or diffusion of gases and their molar masses, stating that lighter gases diffuse more quickly than heavier gases. This principle is crucial in various scientific applications, helping predict how different gases will behave under specific conditions.
Importance of Containment
Given that lighter gases can escape more rapidly, effective containment is essential to prevent potential hazards, such as toxic gas exposure or environmental contamination. Proper containment measures help ensure that gases do not escape into the workspace, maintaining a safe environment for laboratory personnel and preventing unwanted reactions.
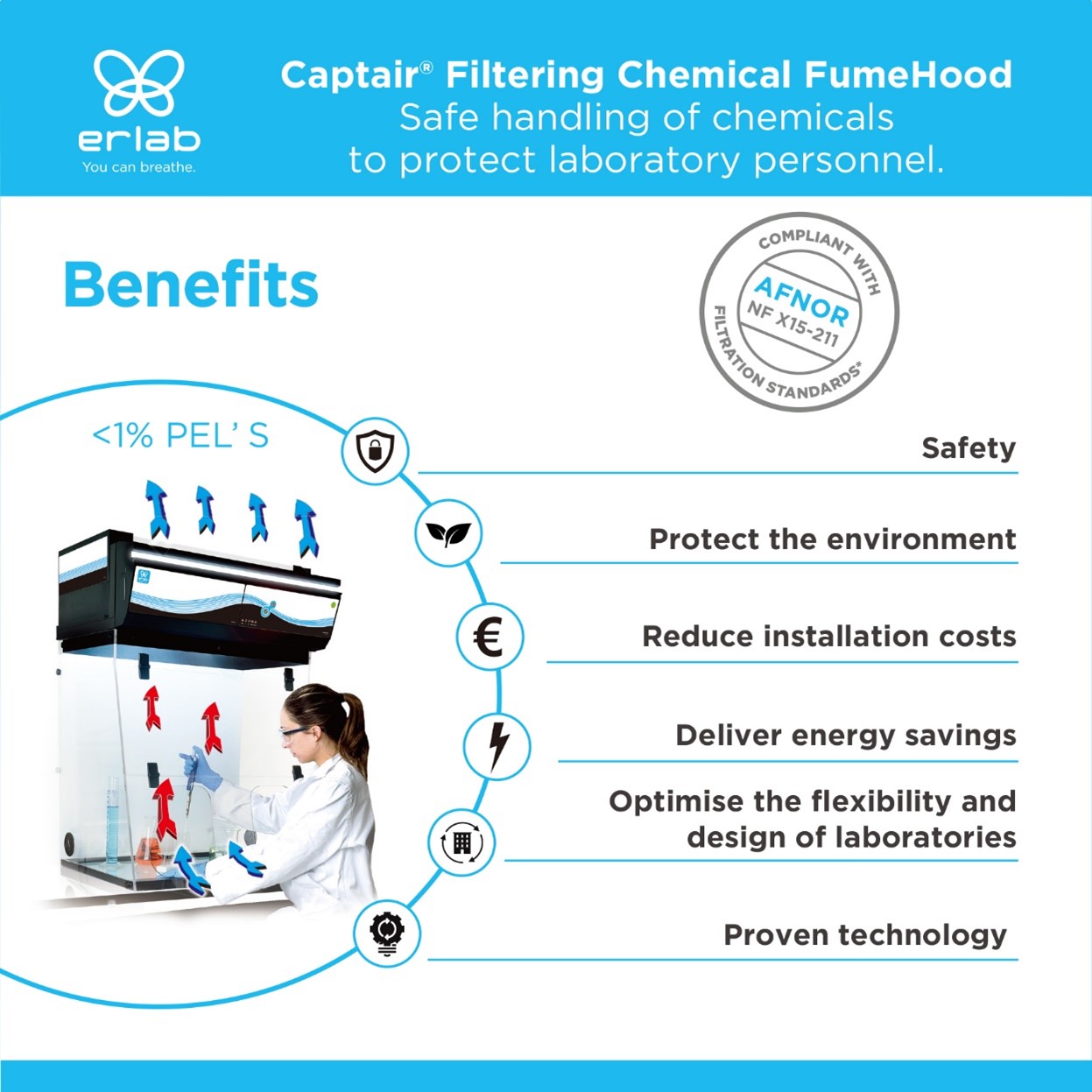
Role of Erlab Ductless Fume Hoods
Erlab ductless fume hoods provide a vital layer of protection for users working with volatile or harmful substances. These fume hoods utilize advanced filtration technology to capture and neutralize harmful fumes and vapors, aligning with Graham’s Law by containing lighter gases that might otherwise diffuse into the surrounding area. By using ductless systems(Erlab ductless fume hood, Erlab instrument enclosure), laboratories can ensure safety and compliance without the need for extensive ductwork, making them an essential resource for safe chemical handling.