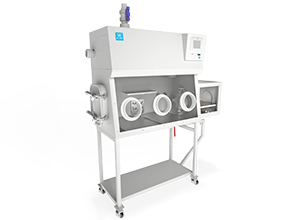
Over the past decade, isolation technology has widely established itself in the field of biomedical research for housing laboratory animals (mice, rats, poultry) as well as in the production processes of injectable drugs both in the pharmaceutical industry and in hospitals.
Isolators, more commonly known as “glove boxes,” are sterile mini-environments that allow substances to be handled in a delimited area that is completely secure for the user. Moreover, operations carried out in a sterile manner in an isolator provide absolutely reliable results.